Biphasic Cuirass Ventilation Global First Feasibility Study
November 17, 2019

UH is the exclusive site to study benefits of device therapy in pediatric Fontan population
Innovations in Congenital Heart | Fall 2019
 Pradeepkumar Charla, MD
Pradeepkumar Charla, MDA global first feasibility and efficacy study is under way at University Hospitals Cleveland Medical Center and UH Rainbow Babies & Children’s Hospital, testing effects of biphasic cuirass ventilation (BCV) in pediatric patients (12-18y) who have had Fontan palliation for univentricular congenital heart disease.
Fontan palliation has become the surgery of choice for children born with single ventricle physiology. During the intricate procedure, surgeons reroute systemic venous blood flow to the lungs, bypassing the need for the subpulmonary ventricle.
With advancements in surgical technique and perioperative care, more babies born with complex congenital heart disease are expected to survive into adulthood. However, in the long run, these patients face unique health challenges. “Adults with Fontan palliation are at high risk for complications secondary to chronic increase in the systemic venous pressure and low cardiac output leading to multi-organ failure,” says Pradeepkumar Charla, MD, Director of the Adult Congenital Heart Disease Program at UH Cleveland Medical Center, and Assistant Professor at Case Western Reserve University School of Medicine. “Patients with failing Fontan physiology often present with symptoms of fatigue, decreased energy levels, rhythm problems and fluid accumulation.”
Effective medical therapies are largely limited to symptom improvement. “The only appropriate option is heart transplantation and is suitable for relatively few patients,” says Dr. Charla. “The idea and search for portable ventilation—a modern iron lung—began during my Pediatric Cardiology Fellowship.”
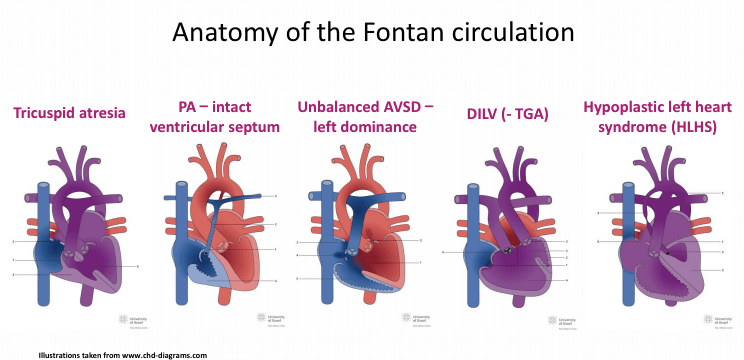 Illustration credit: www.chd-diagrams.com
Illustration credit: www.chd-diagrams.comMAXIMIZING EACH BREATH
The Hayek RTX, manufactured by United Hayek Medical, provides effective, non-invasive ventilation in ambulatory settings. While a patient wears the external cuirass (plastic shield), negative pressure increases airflow to the lungs and positive pressure helps push air back out. “Our research shows that helping patients take maximum breaths improves blood flow to the lungs, which enhances cardiac output,” says Dr. Charla. “We are actually reversing the pathophysiology that leads to Fontan complications. Nothing else we are doing, even surgery, is addressing the underlying cause.”
After demonstrating the safety and positive benefits of BCV in the adult Fontan population, Dr. Charla and his team are studying the safety and efficacy of the external ventilation device in selective adolescent patients. Enrollment criteria for participants in the global first trial include:
•Fontan palliation for complex congenital heart disease, including:
-Tricuspid atresia
-Pulmonary atresia intact ventricular septum (PA-IVS)
-Unbalanced atrioventricular septal defect (AVSD)–left dominance
-Double inlet left ventricle (DILV)
-Transposition of the great arteries (TGA)
-Hypoplastic left heart syndrome (HLHS)
• Age 12-18y
• Currently experiencing no health problems or complications
Study participants wear the BCV device for 10 to 15 minutes as researchers precisely measure pulmonary and cardiac function via cardiovascular flow assessments by echocardiogram and magnetic resonance imaging. “We have shown in adult populations that BCV helps patients overcome the limitations of breath,” says Dr. Charla. “Now we are trying to see if there is the same effect in adolescent patients who have developed comparatively fewer Fontan circuit complications.”
 Hayek RTX
Hayek RTXIMPROVING QUALITY OF LIFE
Similar in weight to a front-facing backpack, the Hayek RTX can be worn while patients perform some of their daily activities. “We hope by wearing the device on a daily basis, the benefit will delay late-onset Fontan complications and improve quality of life,” says Dr. Charla. “Potentially, this device could provide therapeutic support for patients awaiting transplantation or serve as a destination device for people deemed ineligible for transplant. We intend to test these in the next phases of this study.
For more information, or to volunteer or refer a patient for this study, please contact Dr. Charla at 216-553-1537 or email Peds.Innovation@UHhospitals.org.


