Research Compliance
UH Research Compliance is responsible for implementing a program of oversight and auditing that focuses on decreasing risks to the institution, ensuring the safety of research participants, promoting standards of excellence in the conduct of research, identifying and communicating best practices, and ensuring compliance with requirements and ethical standards.
UH Research Compliance will conduct audits or reviews in the form of routine or directed visits at affiliated UH sites, and anywhere the UH IRB serves as the IRB of Record. Research projects are selected by performing a query of the IRB database, reviewing IRB minutes, or may be requested on a voluntary basis by the Principal Investigator, Department, or Clinical Chair.
UH Research Compliance will:
- Assess adherence to Federal regulations as defined by OHRP and FDA
- Assess adherence to UHCMC IRB policies and procedures
- Assess adherence to UHCMC research policies
- Assess adherence to Federal Privacy rule regulations under HIPAA via the Office of Civil Rights
- Assess adherence to local and state laws and regulations
- Assess adherence to regulations as defined by the Office of Research Integrity regarding Research Misconduct
- Determine that the rights and safety of human research participants have been properly protected
- Provide education to investigators
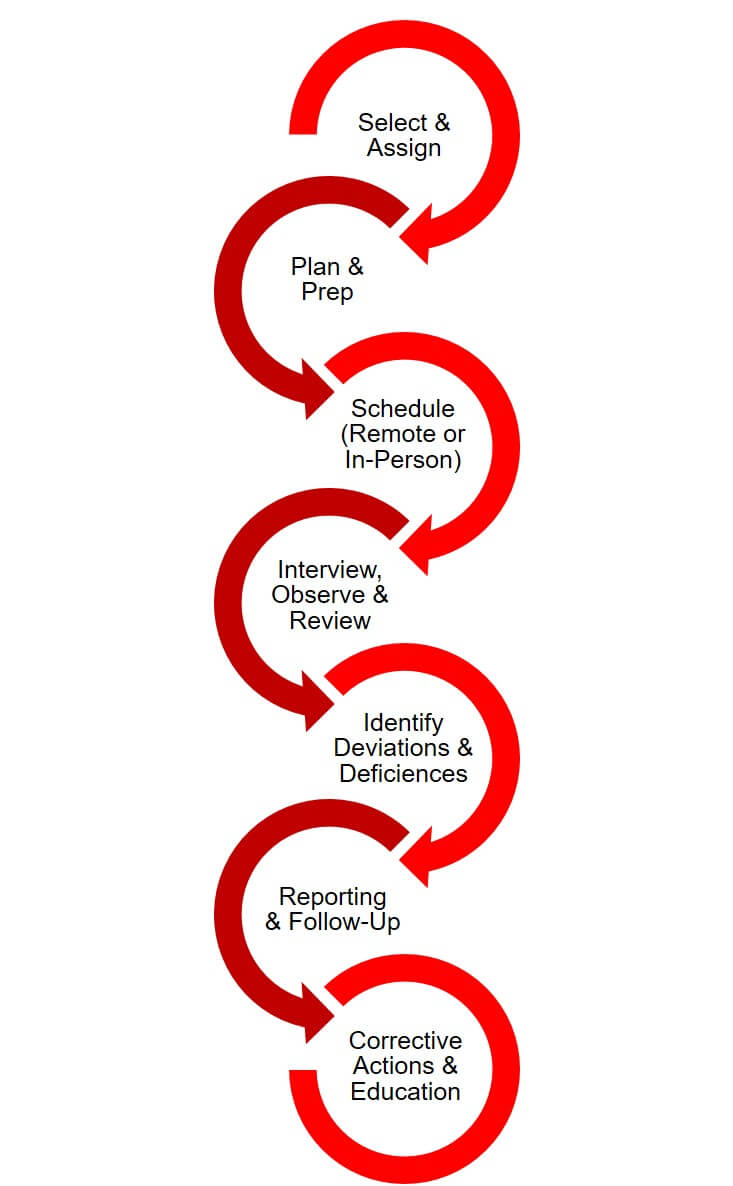
The UH Research Compliance Process
Support Services
- Audit Readiness
- Study Start-Up Consultation
- ICF Observation
- Regulatory Binder Maintenance
- Good Documentation Practices
- Privacy and Confidentiality Use & Disclosures


