Immunotherapy Research & Clinical Trials
The Angie Fowler Adolescent & Young Adult Cancer Institute at UH Rainbow Babies & Children’s Hospital has a rigorous research program with a national reputation for scientific and translational innovation in pediatric oncology and immunotherapy that extends to include teens and young adults (AYA).
The Adolescent & Young Adult Research Initiative at Case Comprehensive Cancer Center at Case Western Reserve University was established in 2014 through the generous $6.7 million gift from the Fowler family to support AYA cancer research, including gaining a basic understanding of the biology of the metastatic process and of therapy resistance in cancers that occur in this age group, and leveraging expertise in imaging and drug development. In 2017, the initiative has awarded funding to numerous projects that hold great promise for developing new and innovative therapies for cancer patients.
Promising projects underway include:
University Hospitals is one of less than a dozen academic medical centers to have successfully manufactured CAR-T cells for human use onsite in our own laboratory and is the cornerstone of the nation's only National Center for Regenerative Medicine.

Employing Immunotherapy Agents Against Sarcoma
Alex Huang, MD, PhD
Leading efforts from UH Rainbow’s new Center for Pediatric Immunotherapy
Evaluating the mechanisms that mediate disease progression
Targeting the VCAM-1/VLA-4 signaling axis in the pediatric and AYA cancers
Survival Benefit for Individuals With CMMRD Undergoing Surveillance
Center of Excellence for Pediatric Immunology and Immunotherapy

The Promise of Immunotherapy for Pediatric Cancer
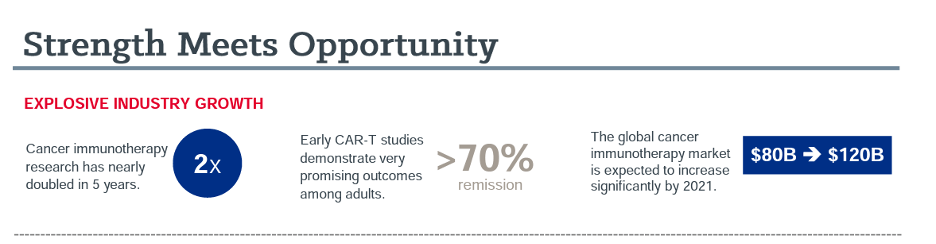
While immunotherapies have gained significant traction in treating adult cancers and are a first line treatment for some cancers, including lung cancer and melanoma, the evolution to pediatric cancers has been slow. Currently, only a few pediatric patients who have not responded to other treatments have access to immunotherapies. However, treatments that work for adults cannot be assumed to work for children. They must be studied through rigorous clinical trials, and the FDA has a high bar for research involving children. The science must be perfect to ensure safety and efficacy, and more data is needed about immunotherapies.
UH Rainbow Babies & Children’s is striving to fill the research gap with a goal to bring the exceptional promise of immunotherapies to our youngest patients fighting cancer. This emerging type of therapy has shown tremendous promise in treating pediatric cancers, providing options for difficult to treat diagnoses and the possibility of reducing toxicities that cause life-long risks for survivors. Immunotherapy has potential to improve outcomes and reduce side effects.


