University Hospitals Recruiting Patients for LeAPPS Prophylactic Stroke Reduction Trial
December 06, 2023
Innovations in Cardiovascular Medicine & Surgery | Fall 2023
University Hospitals Cleveland Medical Center is the only site in Northeast Ohio participating in AtriCure’s multinational, multicenter Left Atrial Appendage Exclusion for Stroke Prevention (LeAPPS™) trial.
 Gregory Rushing, MD
Gregory Rushing, MDThe investigational device exemption (IDE) trial is evaluating the safety and efficacy of AtriClip® for left atrial appendage (LAA) exclusion in subjects undergoing cardiac surgery who have elevated risk for ischemic stroke or systemic arterial embolism and no history of atrial fibrillation (AFib).
“There is a large cohort of patients at risk for stroke or embolism who do not have concomitant AFib,” says Gregory Rushing, MD, Surgical Co-Director, Valve and Structural Heart Disease Center, University Hospitals Harrington Heart & Vascular Institute. “The goal of the trial is to understand whether this population of patients would benefit from prophylactic management of the LAA by applying the AtriClip system.”
Patient Recruitment
The largest randomized clinical trial for LAA exclusion to date, LeAPPS aims to enroll up to 6,500 patients at as many as 250 centers worldwide. The primary endpoint is time to first occurrence of ischemic stroke or systemic arterial embolism or any procedure where the LAA is excluded, occluded or amputated following the index procedure. Study participants will be randomized 1:1 and tracked for a minimum of five years following surgical implantation of AtriClip. Assuming a four-year enrollment period, the trial is expected to be completed at the end of nine years.
Eligible patients include individuals ages 18 and older undergoing planned cardiac surgery who have a comorbidity profile suggesting increased lifetime risk of stroke or embolism. Potential risk factors include increased left atrium size or volume, history of smoking and/or a CHA2DS2-VASc score ≥ 3 with atrial cardiomyopathy.
With more than 45 study participants, University Hospitals is one of the top four sites worldwide for number of patients recruited. “Enrollment is going very well,” says Dr. Rushing. “We have tremendous collaboration among the cardiac surgeons in the division and have developed a robust screening of cases across the system.”
AtriClip LAA Exclusion System
The Food and Drug Administration granted 510(k) clearance to the AtriClip System in 2010 for LAA management of patients with AFib. It is currently the most widely used surgical LAA management system worldwide. Composed of spring-loaded titanium encapsulated in a Dacron outer coating, AtriClip is simple to deploy. Although the device can be placed using a minimally invasive approach, that procedure is not part of the LeAPPS trial.
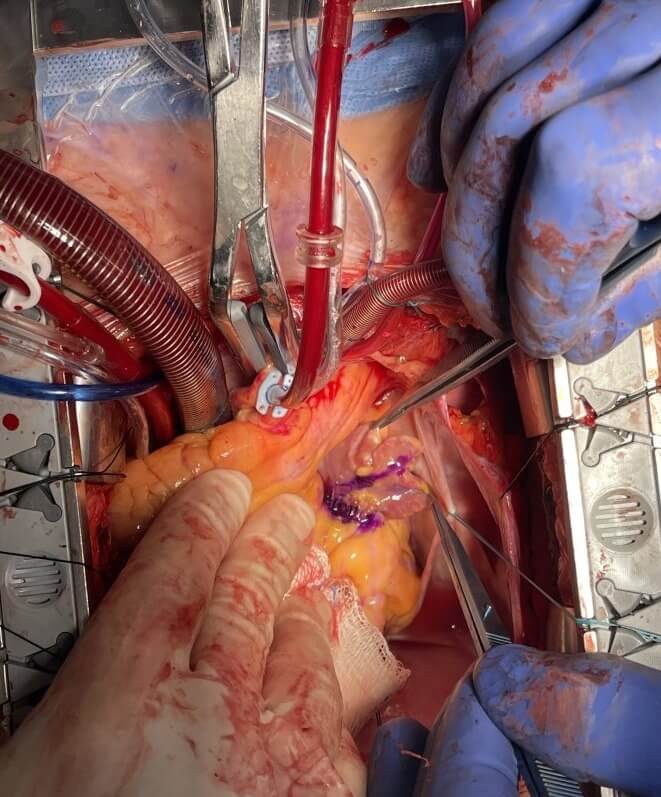 Exposure of the left atrial appendage during on-pump cardiac surgery.
Exposure of the left atrial appendage during on-pump cardiac surgery.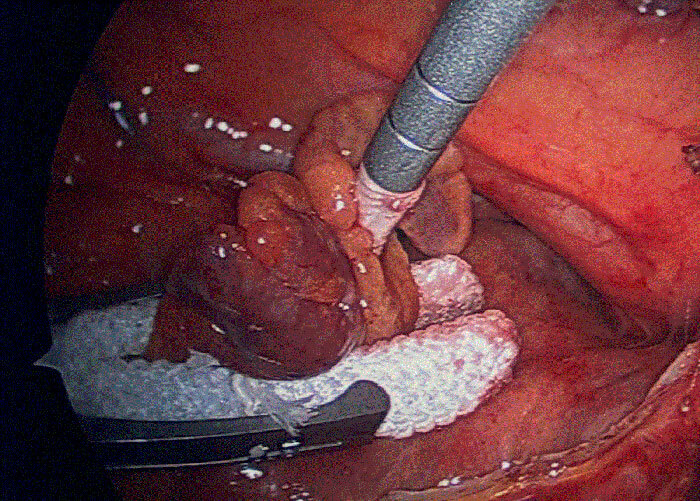 AtriCure Pro-V 35mm AtriClip in place at the base of the left atrial appendage.
AtriCure Pro-V 35mm AtriClip in place at the base of the left atrial appendage.Mitigating Significant Risk
Potentially debilitating stroke or embolism is the most feared complication of cardiac surgery. “We know that the use of this clip has shown in patients with AFib to decrease this risk,” says Dr. Rushing. “Once we have the heart arrested to place a graft or replace a valve, it makes sense that adding this simple, two-minute procedure will provide similar protection to patients who have not experienced AFib.”
Additionally, many patients require anticoagulation therapy. There is always a concern when anticoagulation holidays are indicated in preparation for medical or dental procedures or complications from bleeding. “If these medications need to be held, having the AtriClip in place gives a level of protection and LAA management,” he adds.
A New Standard of Care
Hopefully, the landmark LeAPPS trial will advance best practices for stroke or embolism risk reduction following cardiac surgery. “It is rewarding that we have been asked to participate in this trial,” says Dr. Rushing. “We have the potential to provide the trial-level proof needed to expand the labeled indications and insurance reimbursements for AtriClip and pave the way for a new standard of care in stroke and embolism prevention.”
For more information on the LeAPPS trial or to refer a patient, contact Dr. Rushing’s office at 216-844-4004 or Gregory.Rushing@UHhospitals.org.
Contributing Expert:
Gregory Rushing, MD
Surgical Co-Director, Valve and Structural Heart Disease Center
University Hospitals Harrington Heart & Vascular Institute
Associate Professor of Surgery
Case Western Reserve University School of Medicine


