University Hospitals Team Treats Case of No-Option CLTI with “Off-the-Shelf” Percutaneous Deep Vein Arterialization
December 06, 2023
Patient faced the prospect of a second major amputation
Innovations in Cardiovascular Medicine & Surgery | Fall 2023
University Hospitals Heart & Vascular Institute has been a leader in the clinical trials and ultimate FDA approval of a percutaneous system for deep vein arterialization in patients with chronic limb-threatening ischemia (CLTI). UH Harrington Heart & Vascular Institute president Mehdi Shishehbor, DO, MPH, PhD, Angela and James Hambrick Chair in Innovation, was first author of the NEJM paper and co-principal investigator of the multi-center PROMISE II trial, which found that the minimally invasive system enabled most patients to avoid amputation and experience wound healing.
 Mehdi Shishehbor, DO, MPH, PhD
Mehdi Shishehbor, DO, MPH, PhDHowever, not all CLTI patients qualify for the PROMISE II trial. For such a recent patient with artery-obliterating Buerger disease caused by years of smoking, Dr. Shishehbor and his team recently pushed the bounds of innovation. Using “off-the-shelf,” commercially available devices to perform the deep vein arterialization, they successfully saved the man’s leg from amputation. The team published the groundbreaking case in the prestigious Journal of Vascular Surgery Cases, Innovations and Techniques.
The 55-year-old man presented with right foot rest pain and dry gangrenous second and third toes. His medical history was notable for hypertension, dyslipidemia, chronic active tobacco use (30 pack-years), and Buerger disease and his surgical history for left below-the-knee amputation for gangrene 15 years prior. His femoral and popliteal pulses were palpable, with no discernable pulses in posterior tibial (PT) and dorsalis pedis. The ankle brachial index was 0.62, with a toe brachial index of 0.0.
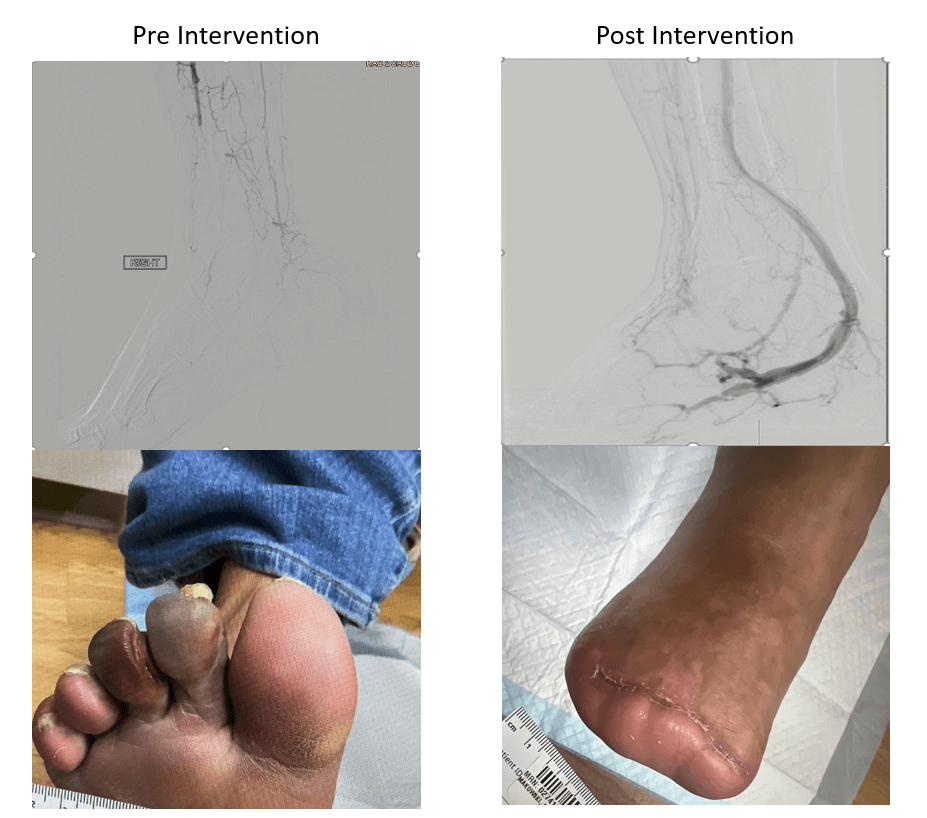
“No named arteries were visualized in the foot or ankle, consistent with “desert foot,” Dr. Shishehbor says. “This patient already had a major amputation on the other side at another hospital a few years ago. He was destined to get another major amputation. We had to innovate above innovation to get a good outcome. By pulling together devices that are already FDA-approved and creating a deep venous arterialization for this patient, we were successful. He did fantastic; he completely healed.”
How was the team able to accomplish this? “The key procedural aspects of percutaneous deep vein arterialization include the use of commercially available reentry devices for establishment of the arteriovenous fistula, balloon venoplasty for valvulotomy, and the use of covered self-expanding stents to line the fistula and outflow venous pathway,” Dr. Shishehbor and the team write. “Although alternative methods have been described for arteriovenous fistula creation, such as double snare technique, bull’s eye technique and spear technique, the use of reentry devices under fluoroscopic or intravascular ultrasound guidance remains the most prevalent technique.”
Even though the team had great success in this case, Dr. Shishehbor says this course of action is not right for every patient.
“Patients should be evaluated for their ability to adhere to the necessary risk factor modifications, such as smoking cessation and medication adherence, as well as wound care regimens, and close clinical and imaging surveillance. Tobacco cessation is of particular importance for patients with Buerger disease, because it is the crux of the disease process,” he says. “Anatomically, patients must have a patent arterial tree down to the proximal tibial level (or a diseased vessel that can be revascularized to provide this inflow pathway and a patent pedal venous arch. The present case report, however, is unique in demonstrating that satisfactory results can be accomplished in the absence of a complete pedal arch via directed venoplasty into alternate available outflow pathways that drain the pedal arch.”
“It will be important for this patient and other patients like him to receive comprehensive follow-up care,” Dr. Shishehbor says. “Close attention to wound healing and routine duplex ultrasound surveillance are paramount after deep vein arterialization.”
However, he says he and his UH Harrington Heart & Vascular Institute team are pleased to report a successful case that may give encouragement to others.
“We’ve shown that percutaneous deep vein arterialization is feasible for properly selected patients with no-option CLTI using devices currently available commercially in an off-label manner,” he says. “Vigilant surveillance and multidisciplinary care are essential to achieve limb salvage after this procedure.”
For more information, contact Dr. Shishehbor’s office at 216-844-5170.
Contributing Expert:
Mehdi Shishehbor, DO, MPH, PhD
President, University Hospitals Harrington Heart & Vascular Institute
Angela and James Hambrick Chair in Innovation
Professor of Medicine
Case Western Reserve University School of Medicine


