Trial of Next Generation Implantable Hypoglossal Neurostimulator to Treat Obstructive Sleep Apnea
May 23, 2023
Innovations in Ear, Nose & Throat | Summer 2023
University Hospitals is the only health system in Northeast Ohio participating in the OSPREY trial, an international study of the LivaNova aura6000™ System, an implantable hypoglossal neurostimulator (HGNS) intended to treat adults with moderate to severe obstructive sleep apnea (OSA).
 Thomaz Fleury Curado, MD, PhD
Thomaz Fleury Curado, MD, PhD“Historically, surgeries for OSA have been aimed at anatomy — removing tissue or expanding the airway. The challenge is that these can be aggressive procedures, and not all patients are accepting of the surgery or good candidates,” says Thomaz Fleury Curado, MD, PhD, an otolaryngologist and craniofacial surgeon within University Hospitals Ear, Nose & Throat Institute. “With implantable pulse generators, the goal is to replenish neuromuscular input to the tongue to maintain muscle tone, which can significantly reduce airway obstruction and apneic frequency.”
HGNS for OSA was developed in animal and human models over the past 30 years to become a viable treatment modality.1 In 2014, the U.S. Food and Drug Administration (FDA) approved the Inspire® Upper Airway Stimulation system for therapeutic use. Inspire utilizes a pacemaker- like implantable neurostimulator to stabilize a patient’s tongue and airway muscles during sleep to prevent obstruction. The device’s components include a pressure-sensing lead that detects patient breathing and a stimulator lead that delivers mild stimulation to the hypoglossal nerve. Before sleep, the patient activates the neurostimulator using a small, handheld remote. University Hospitals was the first health system in Ohio and among the first nationally to offer the novel therapy to their patients.
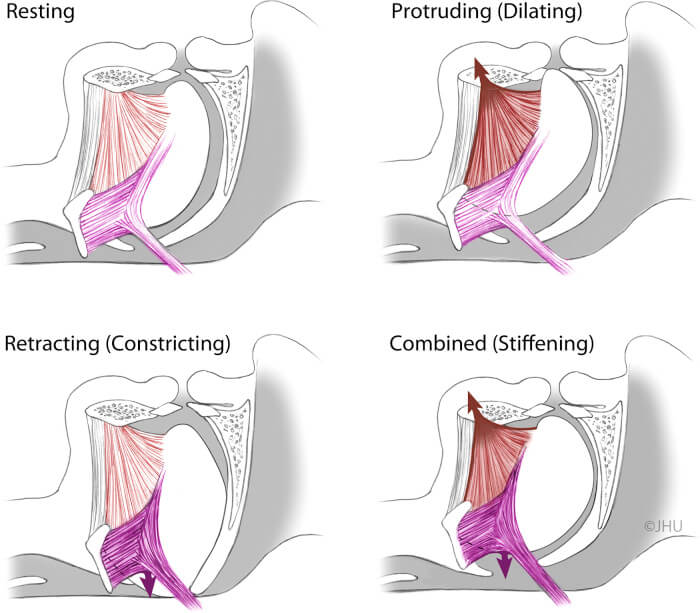 Putative effects of tongue protrudor and retractor stimulation. Upper left panel: resting tongue position and shape. Upper right panel: activation of lingual protrudors markedly dilates the oropharynx. Lower left panel: activation of retractors will lead to active pharyngeal occlusion. Lower right panel: coactivation of tongue protrudors and retractors is superior to protrudors alone in reduction of pharyngeal collapsibility without significant displacement of tongue body. (Illustration by Corinne Sandone, © 2016 Johns Hopkins University, used with permission.)
Putative effects of tongue protrudor and retractor stimulation. Upper left panel: resting tongue position and shape. Upper right panel: activation of lingual protrudors markedly dilates the oropharynx. Lower left panel: activation of retractors will lead to active pharyngeal occlusion. Lower right panel: coactivation of tongue protrudors and retractors is superior to protrudors alone in reduction of pharyngeal collapsibility without significant displacement of tongue body. (Illustration by Corinne Sandone, © 2016 Johns Hopkins University, used with permission.)“Inspire has become an important tool in the arsenal we offer to our patients, but there are some limitations,” says Dr. Fleury. “In patients who receive the surgery and are classified as responders, we see significant reduction in sleep apnea events and improvement in quality-of-life measures, but not all individuals with OSA are candidates for the technology.”
Considerations for Inspire
- To be eligible, individuals cannot have complete concentric collapse at the palate, which must be determined in advance of implantation by observing the airway under light sedation.
- The device must be synchronized with breathing, requiring implantation of a sensing lead to detect inhalation.
- The surgery requires intricate dissection of the hypoglossal nerve, which is responsible for all tongue movement.
Next Generation of Hypoglossal Nerve
The OSPREY trial represents the next generation of HGNS. “We are excited to study the aura6000 in our patients to learn whether this will become another viable option,” says Dr. Fleury. “The concept is to stimulate different groups of muscles — both protruders and retractors — which is sufficient to keep the e Stimulation.
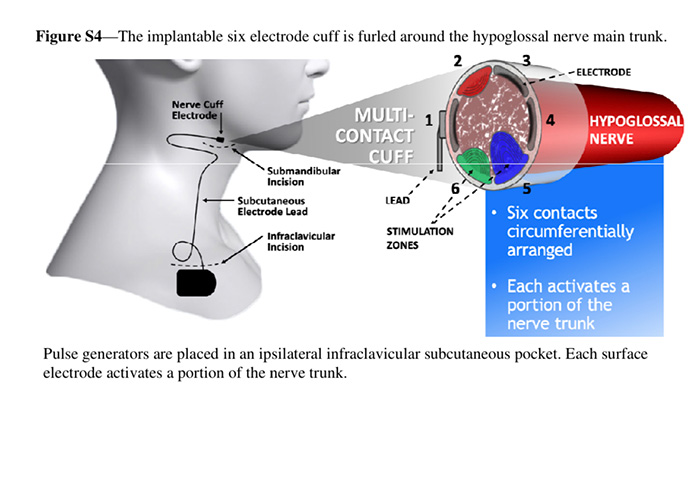
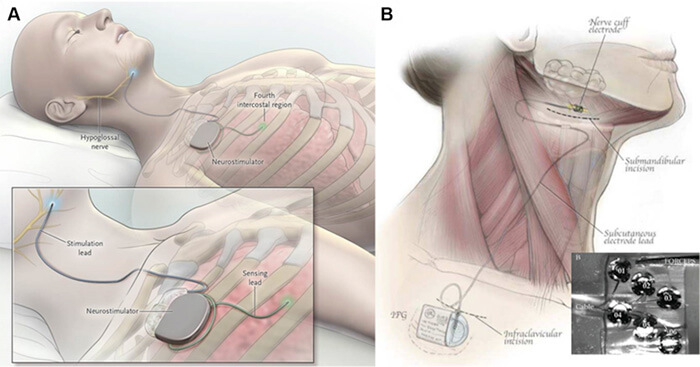 Current systems for hypoglossal nerve stimulation. A, The stimulation lead is distally placed on the medial branch of the hypoglossal nerve to guarantee protrudor selection. The stimulating pulses are delivered via electrodes synchronized with ventilation detected by the sensing lead. B, The implantable six electrode cuff (detail) is furled around the hypoglossal nerve main trunk. Pulse generators are placed in an ipsilateral infraclavicular subcutaneous pocket in both systems. (Adapted with permission from Eisele et al,43 Strollo et al,46 and Zaidi et al.47)
Current systems for hypoglossal nerve stimulation. A, The stimulation lead is distally placed on the medial branch of the hypoglossal nerve to guarantee protrudor selection. The stimulating pulses are delivered via electrodes synchronized with ventilation detected by the sensing lead. B, The implantable six electrode cuff (detail) is furled around the hypoglossal nerve main trunk. Pulse generators are placed in an ipsilateral infraclavicular subcutaneous pocket in both systems. (Adapted with permission from Eisele et al,43 Strollo et al,46 and Zaidi et al.47)tongue in its position and not prone to collapse during sleep.” Surgeons do not need to dissect the hypoglossal nerve, instead placing the sensor on the nerve’s main branch. Additionally, Dr. Fleury explains that the device’s multiple contact points allow stimulation to move across the tongue, helping to avoid fatigue. “The tongue does not fatigue easily, but every muscle or nerve can reach a point of strain,” he says.
Another variation of the aura6000 system is that eligibility for its implantation is not dependent on the absence of circumferential airway collapse. Offered as an outpatient procedure, the device is placed in a subcutaneous pocket near the patient’s clavicle just below the jawbone.
The open-label, prospective OSPREY trial seeks to enroll 150 patients who will be randomized 2:1 in favor of stimulation therapy.
Inclusion criteria:
- 22 and older
- Diagnosed with moderate to severe OSA
- Intolerant of or unwilling to use CPAP therapy
Exclusion criteria:
- Respiratory, cardiac, renal disease or other co-morbid conditions
- BMI > 35 kg/m2
- Specific PSG criteria outlined in the protocol
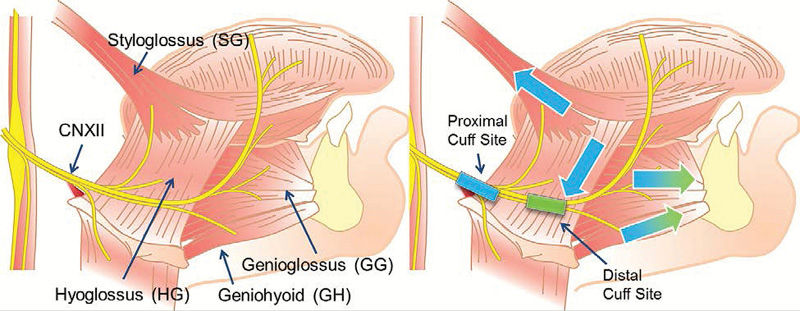 The anatomy and rationale for cuff placement. Left image shows the hypoglossal nerve and its branches to the retractors (styloglossus and hyoglossus) or protrudors (genioglossus and geniohyoid) muscles. Right image shows the two cuff placement sites, where the proximal site results in both group activations while the distal site selectively activates protrudors. Colors denote this difference (proximal results in all four arrows, distal only green arrows)(Reproduced with permission from Schwab et al.57)
The anatomy and rationale for cuff placement. Left image shows the hypoglossal nerve and its branches to the retractors (styloglossus and hyoglossus) or protrudors (genioglossus and geniohyoid) muscles. Right image shows the two cuff placement sites, where the proximal site results in both group activations while the distal site selectively activates protrudors. Colors denote this difference (proximal results in all four arrows, distal only green arrows)(Reproduced with permission from Schwab et al.57)Initial results of the trial show significant reduction in sleep apnea events at month four within the treatment group. “We are fortunate to have one FDA-approved neurostimulator in Inspire, and we expect that upcoming technologies like the aura6000 will continue to drive the innovation of devices for OSA that are less invasive, easier to implant and well-tolerated by patients,” says Dr. Fleury. “At the end of the day, medicine comes down to personalization—an effective treatment for you will not necessarily be the best treatment for me. It is exciting for us as sleep surgeons that we continue to advance treatment solutions that have the potential to improve our patients’ lives.”
For more information or to refer a patient, contact Dr. Fleury Curado at Thomaz.FleuryCurado@UHhospitals.org.
1 Fleury Curado, T., Oliven, A., Sennes, L. U., Polotsky, V. Y., Eisele, D., & Schwartz, A. R. (2018). Neurostimulation Treatment of OA. Chest, 154(6), 1435–1447. https://doi.org/10.1016/j.chest.2018.08.1070
Thomaz Fleury Curado, MD, PhD, Luu Pham, MD, Tamas Otvos, MD, Tracy Klopfer, Carla Freire, MD, Mateus R. Amorim, PhD, Yoichi Nishimura, MD, Luiz Ubirajara Sennes, MD, PhD, Kevin J. Psoter, MD, Mohamed Abdelwahab, MD, MS, Allen Huang, DDS, MD, Raj Dedhia, MD, Stanley Liu, MD, Robson Capasso, MD, Arie Oliven, PhD, Vsevolod Polotsky, MD, PhD, David Eisele, MD, Alan Schwartz, MD. Changes in tongue morphology predict responses in pharyngeal patency to selective hypoglossal nerve stimulation. Journal of Clinical Sleep MedicineVol. 19, No. 5
Contributing Expert:
Thomaz Fleury Curado, MD, PhD
University Hospitals Ear, Nose & Throat Institute
University Hospitals Cleveland Medical Center
Assistant Professor
Case Western Reserve University School of Medicine


