Novel Cardiac Pulmonary Nerve Stimulation Studied at University Hospitals Harrington Heart & Vascular Institute
November 10, 2022
Innovations in Cardiovascular Medicine & Surgery | Fall 2022
University Hospitals Harrington Heart & Vascular Institute is the first program in Northeast Ohio — and one of approximately 25 sites worldwide — participating in an investigational trial of Cardiac Pulmonary Nerve Stimulation (CPNSTM) developed by Cardionomic Inc. The first-in-class treatment was designed for patients who have acute decompensated heart failure (ADHF) with reduced ejection fraction.
 Michael Zacharias, DO
Michael Zacharias, DOMichael Zacharias, DO, Associate Clinical Chief of Cardiovascular Medicine at University Hospitals Cleveland Medical Center and Medical Director of the Mechanical Circulatory Support Program for University Hospitals Harrington Heart & Vascular Institute, sits on the steering committee for the Cardionomic STOP-ADHF trial and is leading the project at University Hospitals. “CPNS is a unique and promising method of optimizing cardiac status,” he says. The non-randomized trial will enroll 90 patients through the end of 2023 and follow them six months past hospital discharge. Dr. Zacharias hopes to enroll five to 10 patients at University Hospitals. Upon completion of the study, safety and performance findings may pave the way for FDA approval.
Though the complications of heart failure are well-defined, treatment options are currently limited. The novel endovascular CPNS system delivers neuromodulation to the autonomic nerves surrounding the right pulmonary artery, stimulating sympathetic and parasympathetic fibers, and increasing left ventricular contractility without the tachycardia associated with inotropes. Under fluoroscopic guidance in a traditional catheterization lab, the device is delivered percutaneously via the internal jugular vein. CPNS has shown potential to improve cardiac function, increase systemic perfusion and support cardiopulmonary decongestion.
The CPNS system includes:
- A neuromodulation stimulation catheter (CN2 catheter)
- A stimulator to deliver electrical current via the CN2 catheter
- An ECG denoiser that receives and passes signals between the patient and monitor
“Implantation of the device is similar to placement of a swan or pulmonary arterial catheter, and a nitinol frame holds it in place,” says Dr. Zacharias. Treatment involves endovascular electrical stimulation of a series of nerves that sits behind the pulmonary artery until the desired response is observed. The catheter can remain implanted for up to five days, after which it is removed, and hemostasis of the venipuncture is achieved.
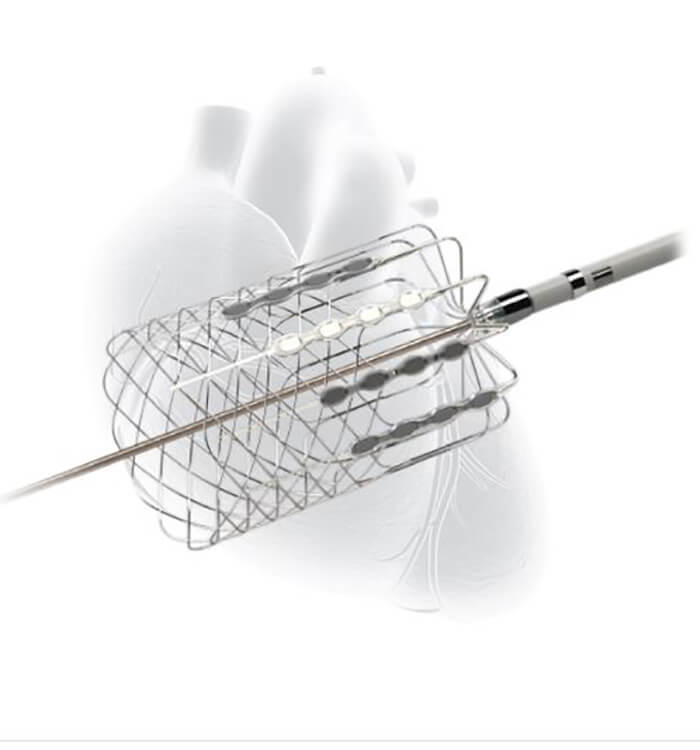 Cardiac Pulmonary Nerve Stimulation (CPNSTM) developed by Cardionomic Inc.
Cardiac Pulmonary Nerve Stimulation (CPNSTM) developed by Cardionomic Inc.Dr. Zacharias explains that improvement of hemodynamic status can stabilize patients enough to increase their tolerance of traditional medications. “The trouble is people can become dangerously decompensated, causing functional limitations,” he says. “We want to capture patients before they have progressed to end-stage heart failure and bridge them physiologically to the point where they can transition to guideline-directed medical therapy [GDMT].”
Inclusion Criteria
“We are looking for patients with a reduced ejection fraction who are admitted with decompensated heart failure, elevated BNP [B-type Natriuretic Peptide] and evidence of volume overload,” Dr. Zacharias says. Other inclusion criteria include:
- Admitted to hospital with a principal diagnosis of ADHF
- BMI adjusted BNP ≥ 500 pg/mL or NT-proBNP ≥ 2000 pg/mL
- Left ventricular Ejection Fraction (LVEF) ≤ 50%
- At least one sign or symptom of fluid overload despite the administration of IV furosemide (or equivalent) (at least 40 mg or equivalent)
Patients who require advanced therapies such as transplant, LVAD, temporary mechanical circulatory support or those with severely reduced renal function are currently excluded from the trial. Additionally, the researchers are not implanting patients who have a defibrillator in place because of concern over dislodging leads.
A Successful Start
Dr. Zacharias and his team at University Hospitals Harrington Heart & Vascular Institute recently implanted their initial patient. “Our first case was a gentleman who was reportedly intolerant of some of the usual medications,” he says. “Following treatment, we were able to explant the device, get him back on his meds and achieve improved cardiac status — he’s doing remarkably well.”
For more information, contact Dr. Zacharias at Michael.Zacharias@UHhospitals.org.


