CorCinch Clinical Trial Underway at University Hospitals
November 24, 2019
Study of AccuCinch® Offers Promise of Novel Non-Surgical Treatment for Enlarged Left Ventricle; Functional Mitral Insufficiency (FMI)
Innovations in Cardiovascular Medicine & Surgery | Fall 2019
 Guilherme Attizzani, MD
Guilherme Attizzani, MD Alan Markowitz, MD
Alan Markowitz, MDOf the estimated 6.5 million adults in the U.S. living with heart failure, approximately half have an enlarged left ventricle, adding stress to the heart wall and decreasing its pumping efficiency. This complicated patient population is notoriously difficult to treat: high surgical risk often outweighs potential benefit and the heart’s decline frequently outpaces current symptom management offered through lifestyle change, medication or cardiac resynchronization therapy. However, AccuCinch®, a first-in-class percutaneous ventricular repair system, is showing promise in a three-part clinical trial, currently underway in the United States and Europe.
Developed by Ancora Heart, AccuCinch is a non-surgical, transcatheter-based implant placed into the left ventricular wall just below the mitral valve. Once cinched properly into place, the investigational device is intended to reduce the size of the left ventricle while providing strength and support to the heart wall.
University Hospitals Cleveland Medical Center is the sole investigative site in Cleveland, Ohio, for the National Institutes of Health (NIH)-funded CorCinch clinical trial of the safety and efficacy of AccuCinch. “Through the aegis of the Valve and Structural Heart Disease Center within the Harrington Heart & Vascular Institute, physicians from both cardiac surgery and interventional cardiology are collaborating to refer appropriate patients to this pioneering trial,” says Alan Markowitz, MD, Chief Surgical Officer and Co-Director, Valve and Structural Heart Disease Center, University Hospitals Harrington Heart & Vascular Institute, Marcella “Dolly” Haugh Chair in Valvular Surgery; Clinical Assistant Professor of Surgery, Case Western Reserve University School of Medicine.
“The goal of the procedure is to reduce functional mitral regurgitation caused by dilation of the left ventricle and improve heart failure symptoms,” says Guilherme F. Attizzani, MD, Co-Director, Valve and Structural Heart Disease Center, University Hospitals Harrington Heart & Vascular Institute, Associate Professor of Medicine at Case Western Reserve University School of Medicine. “As we learn more about the biological response, this novel approach will hopefully improve quality of life for patients who have not had many other options."
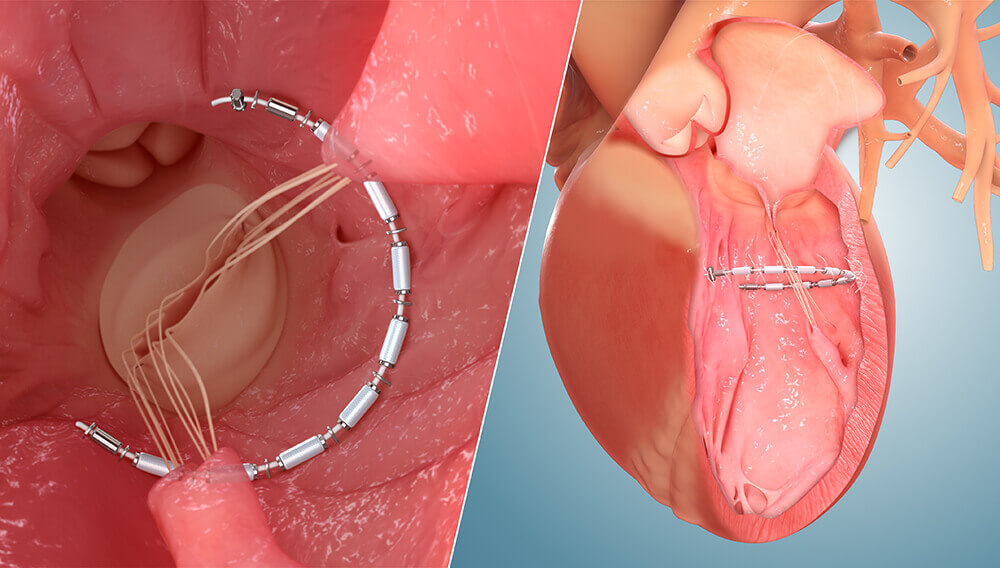 AccuCinch Side-by-side 3D Graphic
AccuCinch Side-by-side 3D GraphicA compelling feature of AccuCinch is that it does not preclude other therapies. “I think a promising aspect of this technology is the combination with MitraClip™ in patients with dilated hearts and severe mitral insufficiency,” says Dr. Attizzani. “Another interesting aspect of the procedure is the ability to treat patients in whom other devices or surgical procedures have failed.”
AccuCinch is the only transcatheter therapy under evaluation for its potential to improve heart function in patients before or after they develop FMR, as well as if the condition has progressed or returned after previous intervention. Other benefits include:
- Direct targeting of left ventricular (LV) dysfunction and dilation
- Percutaneous trans-femoral arterial-retrograde, not trans-septal or trans-apical
- Preservation of natural mitral structures
- Preservation of all future treatment options
Additionally, early clinical data suggests the potential for AccuCinch therapy to support left ventricular reverse remodeling. “As we reduce the size of the chamber and the accompanying mitral insufficiency, we hope to not only improve heart function but also slow disease progression,” says Dr. Markowitz. “This is particularly encouraging because half of all heart failure patients currently do not survive five years past their initial diagnosis.”
Placement of the minimally invasive AccuCinch device occurs in the interventional cardiology lab. The first University Hospitals patient was able to go home after two days of monitoring and is doing well. Study coordinators are currently enrolling patients in two arms of the early feasibility trial:
- The CorCinch-PMVI study of patients with prior mitral valve intervention and recurrent mitral regurgitation
- The CorCinch-HFrEF study of patients with heart failure and reduced ejection fraction
For more information on AccuCinch or patient enrollment criteria, please call 216-553-1439.


