5-ALA: Giving Surgeons an Edge
January 20, 2019
5-ALA helps surgeons visualize boundaries of invading tumors for more complete removal
Innovations in Neurology & Neurosurgery - Winter 2019
In October, a neurosurgeon at University Hospitals of Cleveland’s Seidman Cancer Center was the first U.S. surgeon to use the drug 5-Aminolevulinic Acid (5-ALA) to assist in fluorescence-guided resection of a malignant brain tumor under a new FDA approval.
 Andrew Sloan, MD
Andrew Sloan, MD
Andrew Sloan, MD, who holds the Peter D. Cristal Chair in Neurosurgical Oncology and serves as Director of both the Brain Tumor and Neuro-Oncology Center, and the Center of Excellence for Translational Neuro-Oncology at UH Seidman Cancer Center and UH Cleveland Medical Center; Professor of Neurosurgery, Case Western Reserve University School of Medicine, has conducted clinical trials with 5-ALA for almost a decade via his own FDA-approved Investigational New Drug clinical trial. The drug, which has been the standard of care in most of Europe for many years, causes cancer to fluoresce, which helps surgeons better identify the edges of the tumor and safely remove more of the mass than using traditional visualization methods.
“The goal is maximal safe extraction of the solid portion of the tumor,” Dr. Sloan explains. “With gliomas, you can never take every single cell out, but we know that 99 percent of the tumor is contained in the solid mass. So being able to clearly visualize those boundaries is a real advantage.”
Nearly 16,000 Americans are diagnosed with malignant gliomas each year. Most live fewer than 2 years after diagnosis, but more complete removal of the tumor can extend that.
“It’s very clear that patients do better and survive longer if you can take out as much of the tumor as possible, and any tool that helps you do that helps improve patient outcomes,” Dr. Sloan says.
In fact, patients whose tumors are removed with 5-ALA typically survive twice as long as others, he adds.
LIGHTING THE WAY
Dr. Sloan says his research protocol for using the drug is very similar to what was approved by the FDA last year. Thanks to that approval, he now uses 5-ALA in virtually every applicable case.
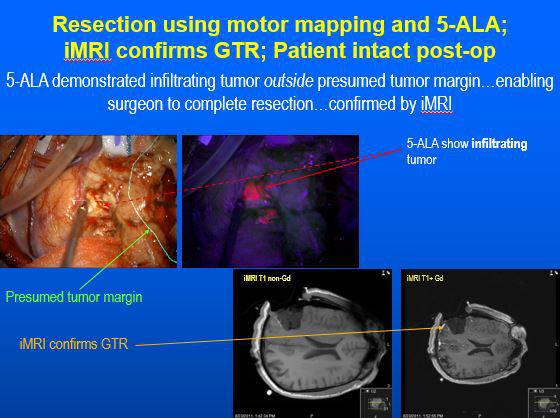
Patients don’t notice much of a difference other than taking the drug by mouth before surgery. Most healthy tissue can metabolize the drug to hemoglobin, a component of blood. However, cancer cells cannot fully process the drug, which accumulates as Protoporphyrin IX and causes malignant brain tumors to glow or fluorescence as pink under blue light. The surgeon uses a specially modified surgical microscope to turn blue light on and off during the procedure to guide the excision. This process only adds a few minutes to the overall surgical time.
“To a large extent, the invading edge of the tumor looks a lot like normal brain,” Dr. Sloan says. “It can be very hard to tell the difference, especially toward the end of the operation when the brain is starting to sag and shift, and the pre-op stereotactic imaging is no longer as precise as it was at the beginning of the case.
“By making the tumor turn pink under the blue light, it makes it easier for the surgeon to know when he or she has the tumor’s boundary. Once you hit that boundary and it’s no longer pink, then you know you need to stop or you risk damaging healthy brain.”
This approach, which Dr. Sloan predicted will eventually become standard of care nationwide, is approved for treatment of Grades 3 and 4 gliomas. He and his colleagues are exploring other indications for it, as well, he says.


