Multiple Myeloma Research
Novel Biologically Based Therapies for Multiple Myeloma
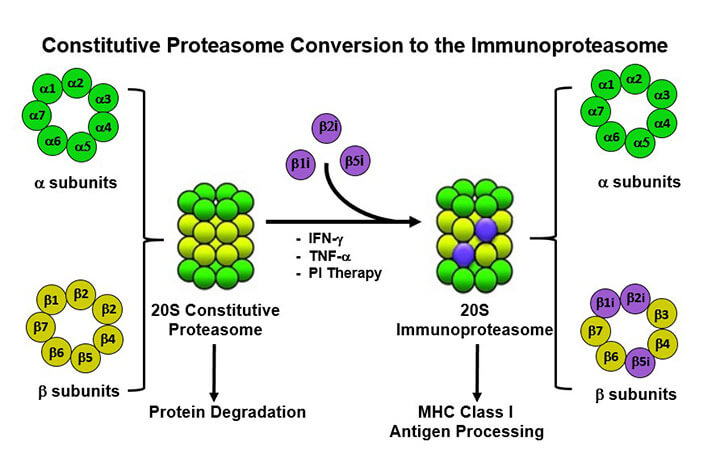 Our NIH-funded (R56, R01) research program is focused on understanding regulation of the proteasome, the most complex, important, and intricately regulated protease in nature. The proteasome exerts regulatory effects over many aspects of cell biology under physiologic conditions and is deregulated in pathologies such as cancer, autoimmunity, proteinopathies, and neurodegenerative diseases. Our long-term goal is to translate advancements in our understanding of proteasomal biology with clinical research and to advance those findings from bench-to-bedside. Despite the arsenal of developed and FDA-approved anti-cancer drugs, cancer is still a leading cause of death. The limiting factor in treating cancers is no longer solely drug availability, but now also includes the need for more comprehensive diagnostic approaches. Personalized cancer care requires treatment that specifically targets biomolecules defining a given tumor, based on knowledge of the tumor’s molecular features. Our recent advances in genomics and proteomics in multiple myeloma (MM) have increased our understanding of disease pathogenesis, helped to identify novel therapeutic targets, and provided the scientific rationale for combining targeted therapies to increase tumor-cell cytotoxicity and abrogate drug resistance.
Our NIH-funded (R56, R01) research program is focused on understanding regulation of the proteasome, the most complex, important, and intricately regulated protease in nature. The proteasome exerts regulatory effects over many aspects of cell biology under physiologic conditions and is deregulated in pathologies such as cancer, autoimmunity, proteinopathies, and neurodegenerative diseases. Our long-term goal is to translate advancements in our understanding of proteasomal biology with clinical research and to advance those findings from bench-to-bedside. Despite the arsenal of developed and FDA-approved anti-cancer drugs, cancer is still a leading cause of death. The limiting factor in treating cancers is no longer solely drug availability, but now also includes the need for more comprehensive diagnostic approaches. Personalized cancer care requires treatment that specifically targets biomolecules defining a given tumor, based on knowledge of the tumor’s molecular features. Our recent advances in genomics and proteomics in multiple myeloma (MM) have increased our understanding of disease pathogenesis, helped to identify novel therapeutic targets, and provided the scientific rationale for combining targeted therapies to increase tumor-cell cytotoxicity and abrogate drug resistance.
The heterogeneous nature of MM makes treating patients with the same drug challenging because finding a druggable oncogenic process common to all patients is not yet feasible. Specifically, gene microarray profiling has shown major differences between normal plasma cells and cells from monoclonal gammopathy of unclear significance (MGUS) and MM cells, with further modulations within MM cells and in cells progressing to plasma cell leukemia. Therefore, we have profiled individual patients newly diagnosed with MM in order to tailor targeted therapy for them; it is likely that cocktails of therapeutics from different classes will be needed to overcome resistance. Recognition of the role of the bone marrow (BM) milieu in conferring growth, survival, and drug resistance in MM cells - in both the laboratory and in animal models - has enabled us to establish a new treatment paradigm targeting the tumor cell and its microenvironment.
Finally, our correlative gene profiling, proteomic, and signaling studies in tumor cell samples from patients treated with novel agents have identified the mechanisms of sensitivity and resistance, provided the rationale for selection of patients most likely to respond, helped to design combination therapies to enhance sensitivity and overcome resistance in MM cells, and suggested ways to develop more potent, selective, and less toxic targeted therapeutics.


