Dermatology Research
Tapinarof for the Treatment of Atopic Dermatitis in Children
This study is currently closed to enrollment.
AGE: 2-17 years old
SEX AT BIRTH: All
SEX AT BIRTH: All
TYPE: Investigational Treatment Study
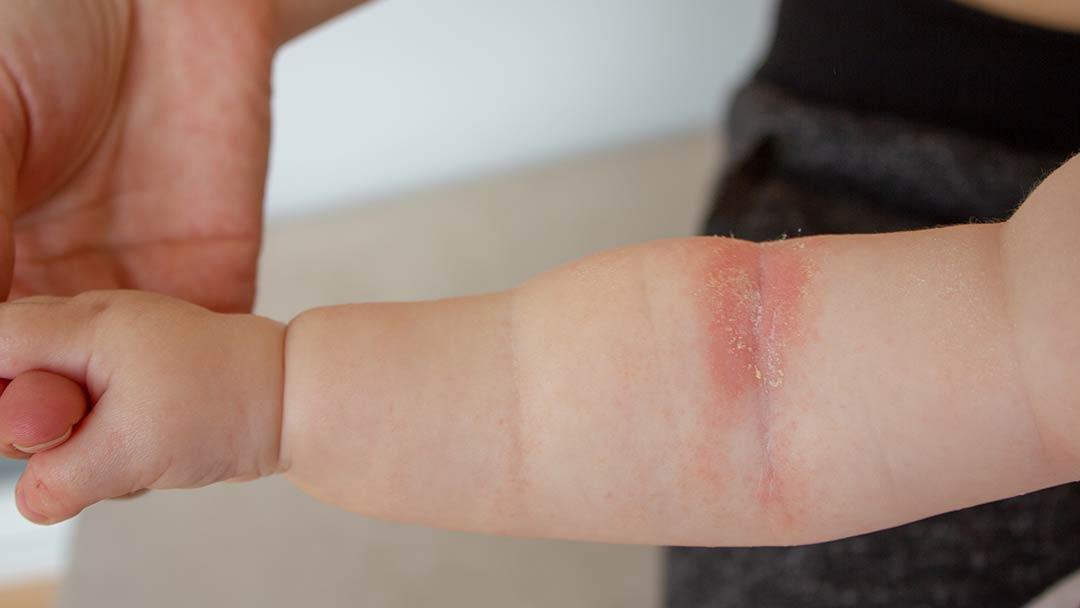
CONDITION: Atopic Dermatitis
CONDITION: Atopic Dermatitis
HEALTHY PARTICIPANTS: No
LOCATION: University Hospitals Medical Centers
LOCATION: University Hospitals Medical Centers
Study Purpose
The purpose of this investigational study is to assess the safety and efficacy (how well it works) of tapinarof cream, 1% compared to a vehicle cream when applied once daily for 8 weeks for the potential topical (on the surface of your skin) treatment of atopic dermatitis.
- STUDY20220598
- NCT05014568


