UH is First Site Activated in Groundbreaking Global Prion Disease Treatment Trial
June 17, 2024
Innovations in Neurology & Neurosurgery | Summer 2024
In a momentous advancement in the fight against prion disease, researchers at University Hospitals Cleveland Medical Center and Case Western Reserve University achieved a historic milestone. They were the first site activated in December 2024 and enrolled the world’s first participant the following month in Ionis Pharmaceuticals’ international, first-in-human study of a prion protein-lowering treatment approach utilizing antisense oligonucleotide (ASO), a synthetic nucleic acid sequence developed to reduce the amount of the normal prion protein.
 Brian S. Appleby, MD
Brian S. Appleby, MD Nicholas C. Bambakidis, MD
Nicholas C. Bambakidis, MDThe trial represents the culmination of several years of collaboration with the pharmaceutical company using data gathered by physician-scientists at the University Hospitals Neurological Institute and the National Prion Disease Pathology Surveillance Center (NPDPSC) at Case Western Reserve University School of Medicine.
The study’s leaders postulate that intrathecal delivery of ION717 will bind to the messenger RNA (mRNA) that encodes normal prion protein so that the body produces less. The double-blind, placebo-controlled trial aims to evaluate the safety and tolerability of the treatment in 56 adult patients with symptomatic prion disease. Currently, there are 11 study sites worldwide.
“The mechanism of action is different from anything that has been tried before,” says Brian S. Appleby, MD, a neuropsychiatrist within the UH Neurological Institute, Professor and Director of the NPDPSC at School of Medicine, and the medical director of the Creutzfeldt-Jakob Disease (CJD) Foundation, a nonprofit advocacy organization supporting families affected by prion disease. “The premise of this study is that reducing the production of normal prion protein will remove the fuel to the fire of the illness,” he says, adding that nonprimate animal models suggest that reducing normal prion protein levels does not contribute to adverse outcomes.
Dr. Appleby is the UH site's principal investigator and collaborated with Ionis for several years prior to the study’s launch. “This is the first truly international trial in our field, which is highly significant for such a rare disease,” he says. “As the first study driven by a pharmaceutical company, this is moving the needle toward future treatment potential and certainly means a lot to patients and families.”
Rare, Devastating and Fatal Disease
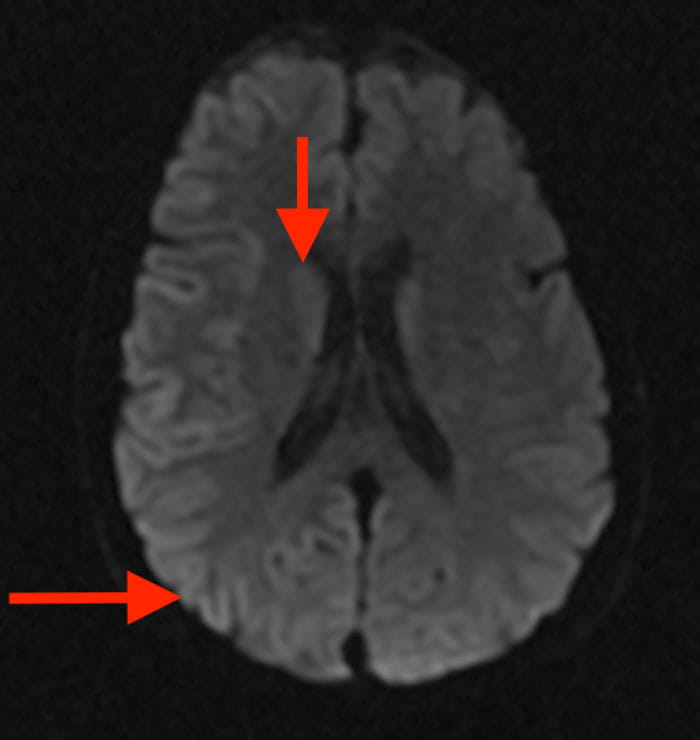 Diffusion weighted imaging brain MRI of a patient with prion disease. High intensity signal is present in the cortical ribbon and caudate heads (red arrows).
Diffusion weighted imaging brain MRI of a patient with prion disease. High intensity signal is present in the cortical ribbon and caudate heads (red arrows).Approximately one to two new cases of prion disease occur per 1 million individuals worldwide, and it is invariably fatal. Also known as transmissible spongiform encephalopathy (TSE), the rare group of progressively neurodegenerative disorders is caused when normal prion proteins misfold and cluster together, resulting in debilitating neurological symptoms that include rapid dementia, unsteady gait, jerky movements and hallucinations. The time from onset of symptoms to death is usually between four and six months.
“While prion disease is only transmittable under very specific circumstances, what separates it from other conditions is that it is known to have three possible etiologies — genetic, acquired or sporadic,” Dr. Appleby says. “Although there are just 500 to 600 new cases of prion disease in the United States each year, we are getting much better at recognizing the disease and diagnosing patients closer to the onset of symptoms.”
Cleveland Hosts the Nation’s Center for Surveillance and Testing
Dr. Appleby’s passion for prion disease research started during his fellowship training at The Johns Hopkins Hospital in Baltimore. In 2017, he became Director of the NPDPSC. Established in 1997 in response to concerns over bovine spongiform encephalopathy (mad cow disease), the NPDPSC is funded by the Centers for Disease Control and Prevention (CDC) and sponsored by the American Association of Neuropathologists.
The only institution of its kind in the U.S., the NPDPSC provides diagnostic testing for prion disease, autopsy coordination, ongoing research, treatment trial development support and resources for families. The center is also the national resource for the Real-Time Quaking Induced Conversion test (RT-QuIC), the only prion-specific antemortem clinical laboratory test of cerebrospinal fluid (CSF) for diagnosing prion disease.
Offering Hope
Under Dr. Appleby’s leadership, University Hospitals is one of three sites nationally and the only site in Ohio participating in this groundbreaking trial.
“The study of ION717 is providing hope for individuals coping with a currently incurable disease,” says Nicholas C. Bambakidis, MD, Chair of the Department of Neurological Surgery, Director and Vice President of the UH Neurological Institute, and Harvey Huntington Brown Jr. Chair in Neurosurgery at School of Medicine. “We are proud to see innovative research conducted at the UH Neurological Institute translate into treatments for patients throughout Northeast Ohio and around the world.”
For more information, contact Dr. Appleby at Brian.Appleby@UHhospitals.org.
Contributing Experts:
Brian S. Appleby, MD
Neuropsychiatrist
University Hospitals Neurological Institute
University Hospitals Cleveland Medical Center
Director
National Prion Disease Pathology Surveillance Center
Professor
Case Western Reserve University School of Medicine
Nicholas C. Bambakidis, MD
Vice President and Director
University Hospitals Neurological Institute
Harvey Huntington Brown, Jr. Chair in Neurosurgery
University Hospitals Cleveland Medical Center
Professor
Case Western Reserve University School of Medicine


